CDC
- A panel of experts is meeting today to advise the FDA on whether to approve Pfizer’s coronavirus vaccine for emergency use.
- They’re also discussing plans for evaluating the safety and efficacy of the vaccine going forward.
- One monitoring system created by the CDC, called V-SAFE, will follow up with vaccine recipients via text if they opt in.
- The V-SAFE health checks are designed to keep tabs on vaccine safety in real time, and any serious concerns will be reported to the CDC and FDA.
- Visit Business Insider’s homepage for more stories.
If the US Food and Drug Administration approves Pfizer’s coronavirus vaccine for emergency use, the collection of safety and efficacy data will continue beyond their current ongoing clinical trial phases.
In a meeting of experts convened today to advise the FDA’s decision, the CDC’s Dr. Nancy Messonnier said monitoring the vaccines as they are rolled out to the public is a “top priority” of the US government.
Messonnier, director of the National Center for Immunization and Respiratory Diseases, described a number of database systems that will help the CDC track any adverse events that could be related to the vaccine.
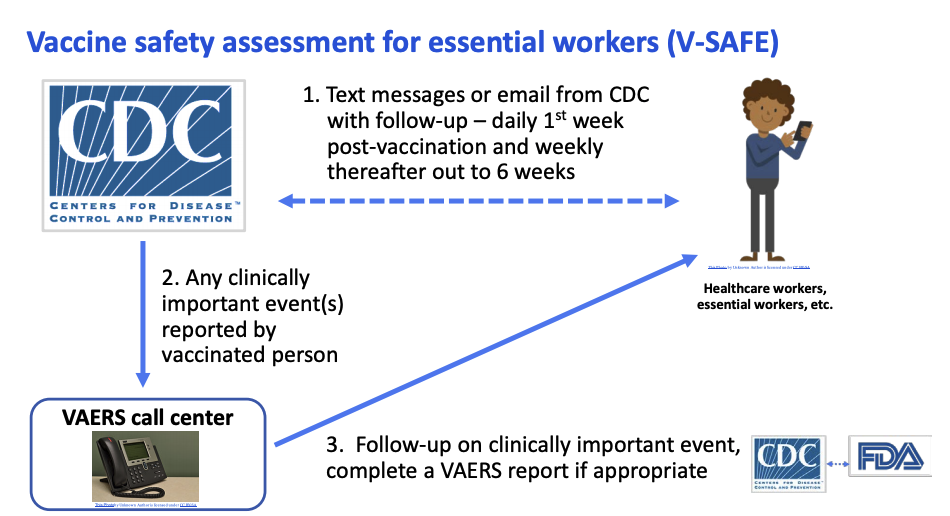
Along with the existing Vaccine Adverse Events Reporting System (VAERS), which is co-managed by the CDC and FDA, the CDC is piloting a new, smartphone-based system to follow early vaccine recipients.
The new safety assessment tool, called V-SAFE, will conduct health checks on vaccine recipients via text messages and emails. People will need to opt in to receive V-SAFE messages for the system to work as intended, Messonnier said in today’s meeting.
"We really need people to sign onto this program to give us the best data possible," she told the committee.
The CDC will follow up with anyone who reports a serious reaction to the vaccine
In previous meetings of the CDC's Advisory Committee on Immunization Practices, Dr. Tom Shimabukuro has explained how the CDC plans to monitor the safety of COVID-19 vaccines in the early stages of rollout.
Vaccine recipients who opt into V-SAFE will get texts or emails daily for a week after each shot. They'll be prompted with weekly check-ins for another six weeks post-vaccination, Shimabukuro said in a September ACIP meeting.
If someone reports a "clinically important" event - symptoms severe enough to interfere with daily activities or merit a doctor's visit - a representative from the VAERS call center will follow up for more information. Scientists from the CDC and FDA will review any VAERS report classified as serious in order to detect any possible safety issues with the vaccine.
So far, two patients have had allergic reactions to the Pfizer vaccine in the UK. Common, temporary side effects of the vaccine reported during the clinical trial include pain at the injection site, fatigue, and headaches.
Other monitoring programs are tailored for long-term care facilities
Residents and staff in nursing homes, assisted living facilities, and other long-term care settings will be among the first to receive a coronavirus vaccine if it is approved.
This group will likely have limited engagement with the V-SAFE system compared to healthcare workers, Shimabukuro said when ACIP met to discuss who should get the vaccine first.
The federal government is rolling out another program that's specifically geared toward long-term care facilities. The system, created by the National Institutes of Health, Brown University, and Genesis HealthCare, will monitor roughly 35,000 long-term care residents at 250 facilities across the US, Messonnier said today.